M201-A
Drug for heart failure, atrial fibrillation and kidney disease
A novel therapeutic agent for heart failure, atrial fibrillation and chronic kidney disease, ryanodine receptor 2 inhibitor, M201-A

Ryanodine receptor 2 (RyR2) exists in the membrane of intracellular endoplasmic reticulum (ER) in the heart, kidney, pancreas and other organs and plays a crucial role in regulating intracellular calcium ion (Ca2+) levels and maintaining the homeostasis of cellular functions. It has recently become clear that abnormal Ca2+ leakage from the ER into the cytoplasm due to catecholamines, oxidative stress and ageing can cause heart failure, atrial fibrillation and renal dysfunction. M201-A is a RyR2 inhibitor that suppresses abnormal Ca2+ leakage from the ER and might be an innovative drug for treating heart failure, atrial fibrillation, renal dysfunction and other diseases.
Phase I, single and repeated clinical trials for the injectable form have been completed, and Phase II clinical trials are underway for patients with heart failure and renal dysfunction.
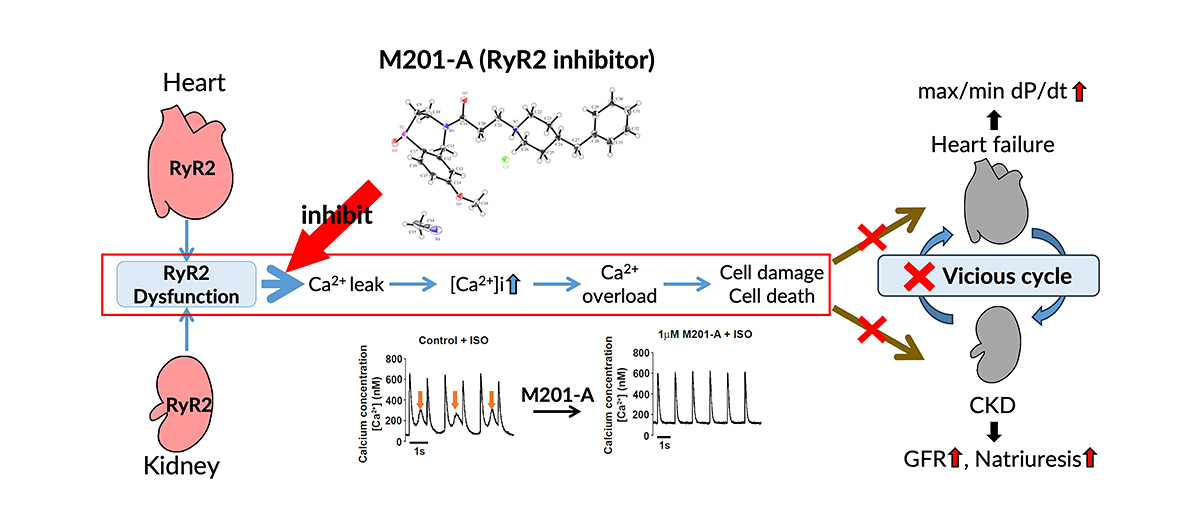
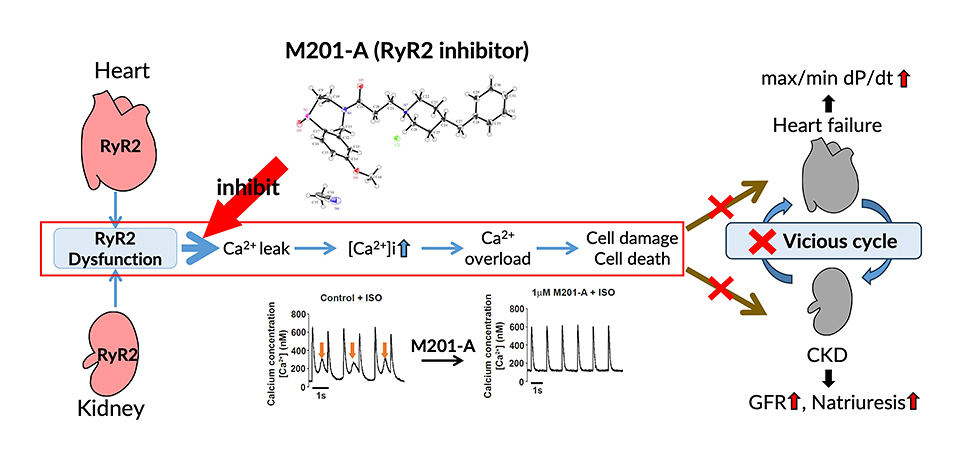
Kaneko N, Loughrey CM, Smith GL, et al. A novel ryanodine receptor 2 inhibitor, M201-A, enhances natriuresis, renal function and lusi-inotropic actions: Preclinical and phase I study. Br. J. Pharmacol., Published online 2024. DOI: 10.1111/bph.16379

K201 was developed as an intracellular Ca2+ inhibitor using the Ca2+ overload model with explanted hearts as a screening system. Subsequently, it was found to improve RyR2 dysfunction and was effective against experimental heart failure, arrhythmia, kidney disease and diabetes mellitus. Phase I and Phase II clinical trials for injectable and oral formulations are underway in the United States and Europe.
Significantly reduces urinary frequency and residual urine. Under development for treating overactive bladder.